Spend enough time researching injectable fillers, neurotoxins (BOTOX & Dysport), and other non-surgical treatments, and eventually you’ll come across this term: “off-label.” As is the case with medical jargon, “off-label” use is a term tossed around by providers without much thought; however, as a patient, you may see it and wonder, “what the heck does that mean?” and more importantly, “is it safe?!”
Well, we’re going to answer that question for you, plus give you helpful info to make more informed decisions about your treatment.
First of all, “off label use” isn’t as scary as it sounds.
While using something “off label” may sound iffy, it’s actually quite common (studies show an estimated 20% of prescriptions are used for off-label purposes) and can be very safe and effective in the appropriate situation.
“Off-label” product use is common and safe and effective in the appropriate situation. The skill and experience of the provider administering treatment is the most important factor for safe treatment and great results.
“Off-label” refers to the use of a food, drug or medical device for an area or issue that isn’t yet advertised to treat. A drug company can only advertise a product for the intended uses that the FDA has officially approved. For example, take a look at this snippet from botox.com. We’ve circled the FDA intended use language in red:
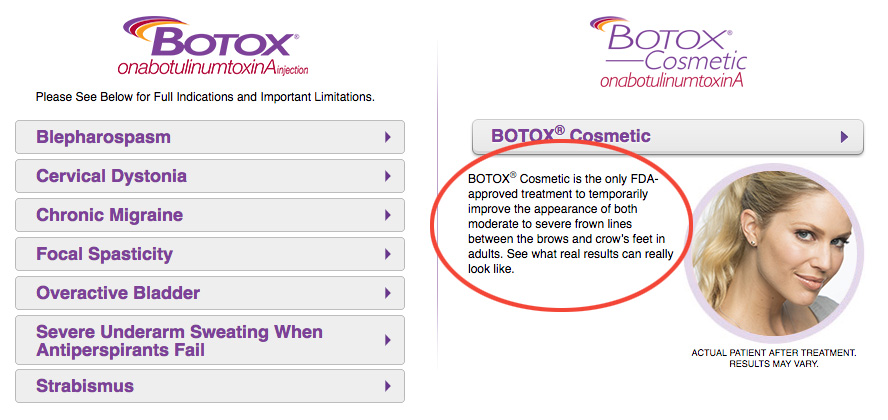
This does not mean that the uses on the label are the only safe and effective uses for that product—there may be dozens of other safe and effective ways to use a product that the company is not yet allowed to advertise. It is also completely legal for your physician to offer an off-label treatment if they feel it is appropriate for you. In fact, off-label use of one product is in many cases a better option than an on-label use of another.
The FDA approval process in a nutshell (why it takes so long to get on the label)
The FDA regulates the availability of a drug or medical device and its claims. When a company applies for FDA approval of a product, they must submit specific use information to be included on the label (which can then be posted on the company’s website and advertisements) along with testing and manufacturing data. You can read through the basic steps here.
Here’s the kicker: the FDA requires a separate approval process for each intended use of a product, including resubmission of clinical data and other information, which can take years to complete each go-around. Therefore, products in the cosmetic medicine field are typically presented with one specific use to get the ball rolling.
For instance, BOTOX® Cosmetic was FDA approved only to treat frown lines from 2002 to 2011. However, physicians were already using it safely and successfully for crow’s feet, brow furrows, and other issues during this period. Doing so not only allowed them to help more patients sooner, but also allowed BOTOX’s manufacturers to generate the resources needed to get these additional uses on the label. CoolSculpting is an example of a medical device with a similar history. For several years it was only FDA cleared to reduce fat on the abdomen and flanks, although physicians also used it to reduce thighs, buttocks and bra rolls (it’s now FDA cleared to treat these areas too).
On- or off-label, choose a qualified, experienced provider
Ultimately, whether a product is used exactly as advertised or off-label matters less than the skill and experience of the person administering your treatment. So be sure to choose a board certified plastic surgeon or other experienced, licensed medical professional working under physician supervision. A reputable provider will know how a product can be used safely off-label—and how it shouldn’t be used—and will only recommend a treatment that is appropriate for your needs.
More tips to ensure your safety:
- Don’t hesitate to ask questions about products or a provider’s experience or methods. Doctors are not legally obligated to tell you if a treatment is FDA approved; a reputable provider will be forthcoming in his/her answers.
- Beware of products or treatments being sold for a considerably lower price than normal or providers who won’t answer questions about the product. These are red flags that the product may be a knockoff, or a substance that has not been vetted for safety and efficacy.
Have questions? We’re ready to help.
If you’d like to learn more about safe, effective cosmetic treatments for your face, skin and body, come see us at LJC! Our experienced La Jolla Cosmetic Medical Spa team and board certified plastic surgeons take patient safety seriously. We will be glad to explain to you how we use FDA approved products and treatments off-label and help you choose the best treatment options for your needs at a free consultation.
